
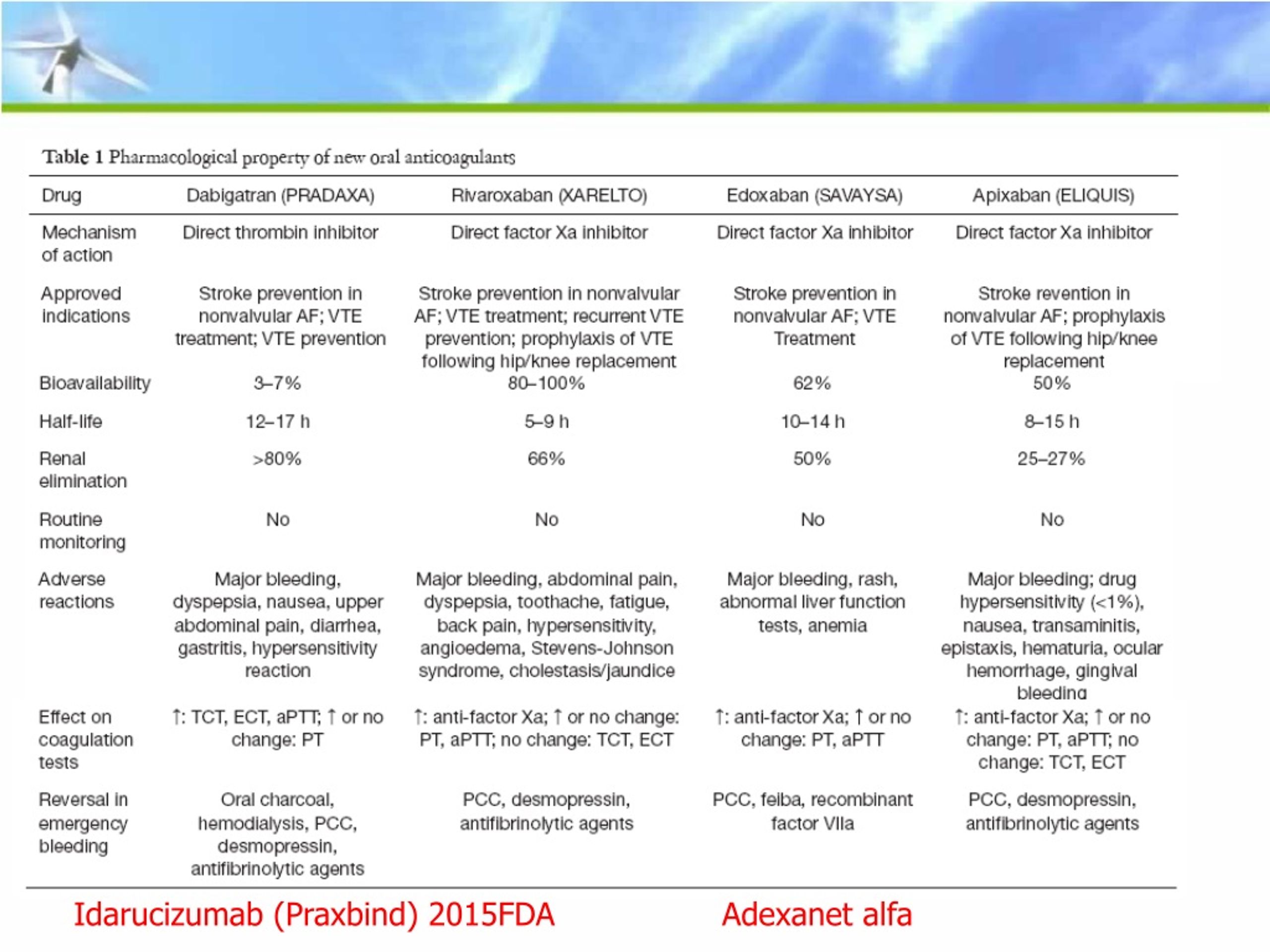
Computer alerts and the use of tall man letters for computer selection screens (idaru CIZUmab and IDArubicin) should also be considered for these drugs. (There is already a risk of bleeding in many oncology patients undergoing treatment.) Once an order is entered electronically, barcode scanning of the drug vials will prevent a mix-up if it’s done prior to sterile compounding. A vial of idaru CIZUmab was spiked with a closed system transfer device, but fortunately, a pharmacist noticed the error before any drug was actually administered.Īs noted, many hospitals may already stock or are planning to stock idaru CIZUmab in case it is needed, although cancer hospitals may not see a need to stock the drug. The idaru CIZUmab carton was in the refrigerator next to a bag containing IDArubicin, which was being readied for a patient. The hospital had just purchased the reversal agent when the close call incident occurred. This past week we received a report of a close call at a hospital where idaru CIZUmab was pulled from stock instead of IDArubicin. This might lead to the selection of the wrong drug from a computer system dropdown menu or selection of the wrong container from its storage location, since both drugs are refrigerated solutions. Unfortunately, the drug’s nonproprietary name, idaru CIZUmab, shares its first five letters with the antineoplastic drug IDArubicin. Hospital pharmacies may stock Praxbind if dabigatran patients are treated in the hospital or seen in the emergency department.


Last month the US Food and Drug Administration (FDA) granted accelerated approval to PRAXBIND (idarucizumab) for use in patients who take PRADAXA (dabigatran) and suffer a life-threatening bleeding event.


 0 kommentar(er)
0 kommentar(er)
